A Drug to Forget: PTSD Memory Disruption Achieved with Single Dose in Female Mice
In a striking new study published in Brain Medicine (Genomic Press, New York), neuroscientists report that the drug Osanetant, when administered shortly after trauma, significantly reduced fear memory in a PTSD-like model in female mice. The peer-reviewed study offers an important preclinical step toward gender-specific interventions for post-traumatic stress disorder.
This research, presented as a peer-reviewed Brevia article and already attracting early attention from psychiatric researchers, taps into an under-explored neurochemical pathway—the Tachykinin 2 (Tac2) system—and demonstrates a novel method for dampening traumatic memory formation through Nk3R antagonism.
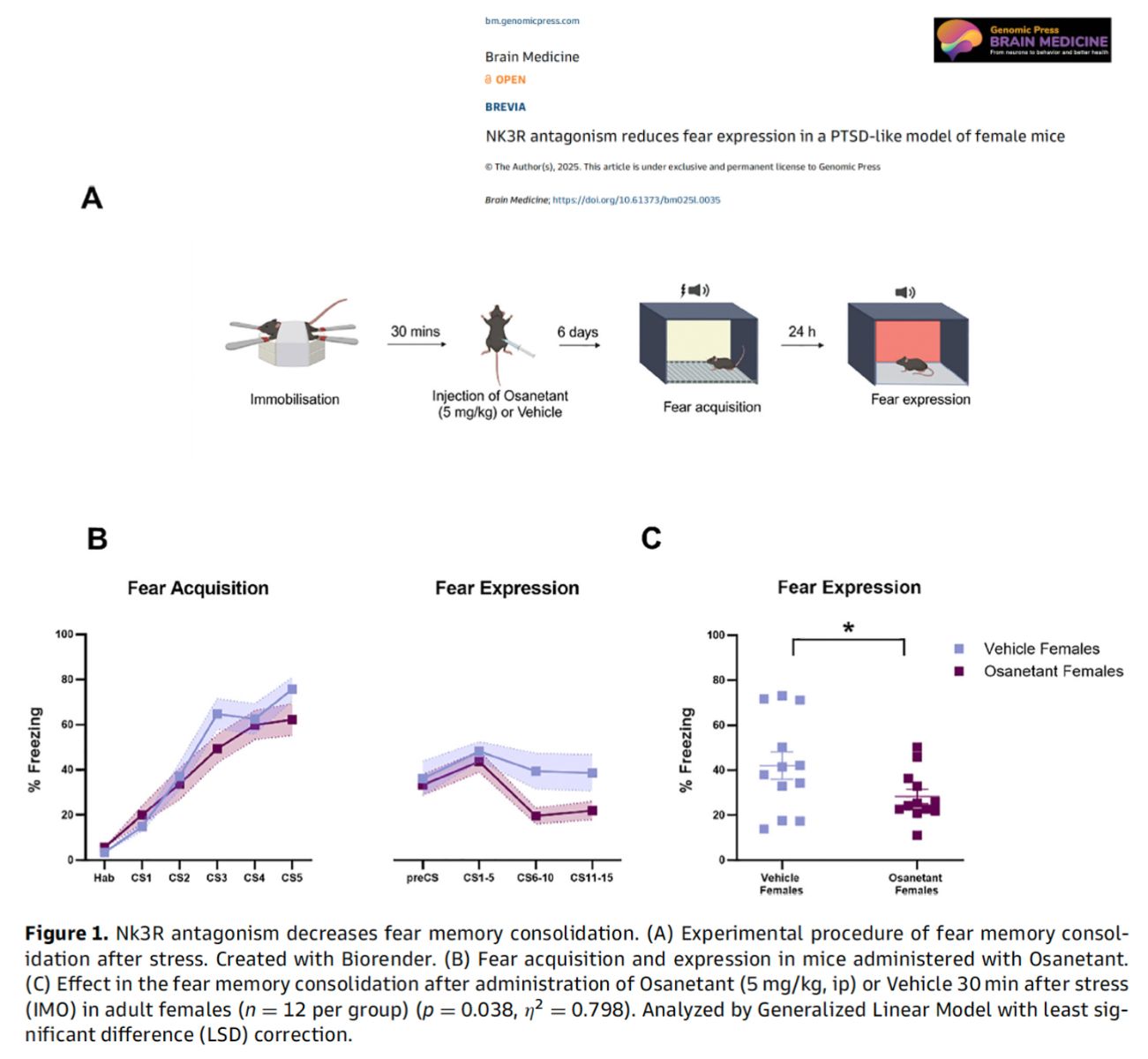
Figure legend: Diagram showing the timeline and behavioral impact of Osanetant administered 30 minutes after immobilization stress. Mice treated with the drug froze significantly less in fear-response tests six days later, suggesting impaired consolidation of fear memories.
Study Summary
The study’s core findings include:
• A single systemic dose of Osanetant (5 mg/kg) administered post-trauma led to reduced fear expression in female mice, mimicking potential early intervention in PTSD.
• This effect was specific to fear memory consolidation, with no change in the initial fear acquisition phase.
• The observed reduction was statistically significant (p = 0.038) and had a large effect size (η² = 0.798), according to the authors' general linear model analysis.
• The findings contrast with earlier reports showing opposite effects of the same drug in non-stressed mice, suggesting context-dependent modulation based on prior trauma exposure
Fear, Sex Differences, and the Tac2 Pathway
PTSD affects women at twice the rate of men—yet female subjects remain vastly underrepresented in both human and animal fear memory research. This study explicitly addresses that gap, employing a PTSD-like immobilization stress model in adult female mice and zeroing in on the Tac2 gene, which encodes Neurokinin B (NkB).
NkB binds to the Neurokinin 3 receptor (Nk3R), a molecular gateway to neural regions involved in fear processing, such as the centromedial amygdala, hippocampus, and bed nucleus of the stria terminalis. Previous research hinted at the Tac2 system’s role in stress response; this study confirms its real-time impact on memory when blocked pharmacologically.
Implications for Trauma Care
Lead researcher Dr. Raül Andero of the Universitat Autònoma de Barcelona suggests these findings could open new preventive strategies for PTSD:
“Administering Osanetant immediately after trauma exposure—say, in cases of sexual violence or car accidents—might one day help prevent pathological memory consolidation in women.”
Yet the study also raises pivotal questions:
- Could this gender-specific effect be replicated in humans?
- Is there a narrow post-trauma window during which Osanetant is effective?
- What is the molecular logic behind the opposing effects of the same drug in stressed versus non-stressed subjects?
Limitations and Future Directions
This study did not monitor the estrous cycle, excluded male subjects, and was limited to behavioral outcomes. However, it builds on prior findings showing that stress intensity may rewire synaptic plasticity pathways, including BDNF, GSK-3β, and β-catenin—opening a door to molecular exploration of stress-modulated fear consolidation.
While Osanetant has previously shown tolerability in humans, its potential clinical application in PTSD prophylaxis requires rigorous follow-up, especially to decode the interaction between stress, sex, and memory circuitry.
Publication Details
The peer-reviewed article appears in Brain Medicine, providing valuable context about Genomic Press's mission to support innovative, cross-disciplinary research bridging fundamental neuroscience and translational initiatives in brain medicine. The journal's unique scope encompasses the underlying science, causes, outcomes, treatments, and societal impact of brain disorders across all clinical disciplines and their interfaces.
🔓 Open Access
Peer-reviewed Brevia: https://doi.org/10.61373/bm025l.0035
Social Media Links to the Brevia:
- 🔗 LinkedIn: https://lnkd.in/d68aHe5r
- ✖️ (Twitter): https://x.com/GenomicPress/status/1912222426911633504
- 📸 Instagram: https://www.instagram.com/p/DIeqVBjJMJZ/
- 📘 https://www.facebook.com/share/p/18j6jTSCRG/
- 🦋 Bluesky: https://url.genomicpress.com/2p93s7tt
Press Coverage:
- 🇬🇧 EIN Presswire in English (EN): https://url.genomicpress.com/2p85hcmn
- 🇫🇷 EurekAlert! press release in English (FR): https://url.genomicpress.com/2p8umsdj
- 🇪🇸 EurekAlert! press release in Spanish (EN): https://url.genomicpress.com/2p837n6m
- 🇨🇳 EurekAlert! press release in Chinese (ZH): https://url.genomicpress.com/2p9fzdtp
Global Impact of the Brain Medicine Brevia Article:
🇺🇸Scienceblog.com (USA): https://url.genomicpress.com/mhs5ydc
🇺🇸Science News Today (USA): https://url.genomicpress.com/4zmden7v
🇬🇧News-Medical.net (UK): https://url.genomicpress.com/2p8v56mc
🇬🇧Medical Xpress (UK): https://url.genomicpress.com/2xjkssr8
🇬🇧FirstWord Pharma (UK): https https://firstwordpharma.com/story/5948146
🇦🇺Mirage News (Australia): https://url.genomicpress.com/5x57y8h
🇫🇷 Santé log (France): https://url.genomicpress.com/2p8cx7p6
🇪🇸La Razón (Spain): https://url.genomicpress.com/59r6fvsb
🇪🇸Biotech Spain (Spain): https://url.genomicpress.com/28frjpwe
🇪🇸El Periódico (Catalonia, Spain): https://url.genomicpress.com/59r6fvsb
🇪🇸RAC1.cat (Catalonia, Spain): https://url.genomicpress.com/434ae3fy
🇨🇳ebiotrade.com (PRC): https://url.genomicpress.com/3anydfa2
This paper exemplifies Brain Medicine's emerging role as a leading international forum for critical public health research. Within days of publication, the study's findings were rapidly disseminated across the world.